Artificial Intelligence is often described as a game-changer for healthcare and medical technology. Yet in practice, many organizations are still experimenting on the margins. PoCs are launched, pilot programs are explored, and innovation labs publish concept demos, but AI is rarely embedded across the entire product lifecycle.
That hesitation is a missed opportunity.
In MedTech, the stakes are uniquely high. Products must be safe, compliant, reliable, and clinically meaningful. At the same time, the industry is under pressure to innovate faster, reduce development costs, shorten regulatory cycles, and deliver more personalized care. Traditional development methodologies struggle to meet those competing demands.
AI is not just another tool in the engineering stack. It is rapidly becoming the foundational layer enabling smarter design, faster validation, predictive insights, and connected product ecosystems.
At Pinetics, we work closely with device manufacturers, healthcare technology innovators, and life-science companies to embed AI into Embedded Product Development Services, Hardware Design and Development, Electronic Product Design Services, and Medical Device Hardware Design. AI is no longer optional; it is the differentiator between incremental improvement and market-leading transformation.
Beyond Pilots: Why MedTech Must Move Past “Experiment Mode”
Most industries’ MedTech included are still stuck in pilot mode. AI is explored in isolated phases, such as data analytics or diagnostics automation, while the rest of the product lifecycle remains unchanged.
True transformation only occurs when AI is implemented across:
- Early-stage research and concept development
- Hardware and electronic product design
- Embedded software and firmware architecture
- Regulatory strategy and verification
- Post-market surveillance and lifecycle management
Medical technology companies that embed AI systematically can:
- Accelerate innovation cycles
- Reduce trial and prototyping costs
- Anticipate failures before they occur
- Create adaptive, learning devices
- Improve patient outcomes through personalization
AI is not replacing engineering; it is augmenting it. Engineers still design systems, but AI expands what is possible in design space, data interpretation, and automation.
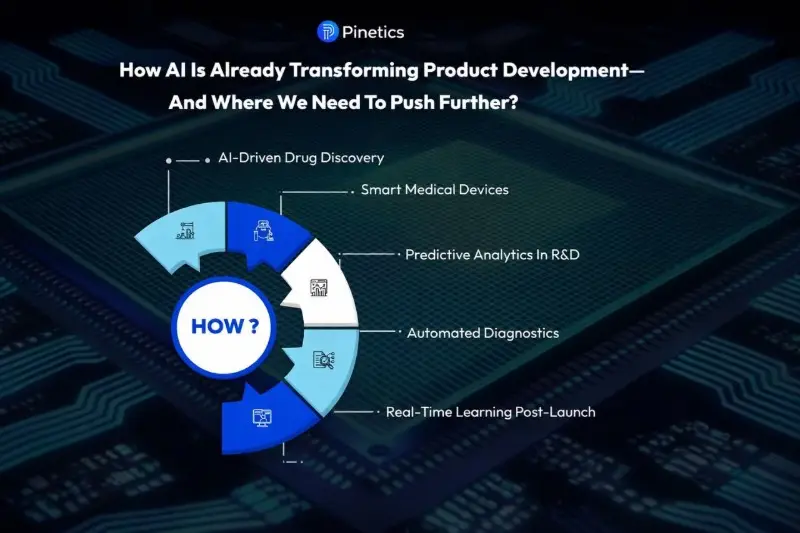
Real-World Examples: How AI is Already Changing MedTech Product Development
The shift is already underway. A number of leading organizations demonstrate what is possible when AI moves beyond PowerPoint concepts and enters the product pipeline.
AI-driven Drug Discovery
AI dramatically accelerates the discovery of therapies by analyzing vast biological datasets and identifying hidden relationships.
For example, advanced AI models can evaluate patterns in genes, proteins, and cellular pathways to predict candidate molecules far more efficiently than traditional lab iteration cycles. Companies such as Verge Genomics analyze human tissue data at scale to reduce drug discovery timeframes for neurodegenerative diseases like ALS.
This level of acceleration fundamentally changes the R&D economics for pharmaceutical and MedTech hybrid organizations.
Smarter, More Adaptive Medical Devices
Generative AI design systems are transforming hardware engineering.
Generative algorithms evaluate millions of structural, ergonomic, and material configurations in hours; something impossible using manual CAD alone. Companies like BioSerenity leverage AI to optimize wearable healthcare devices, making them more precise, lightweight, and patient-friendly.
This is directly linked to Electronic Product Design Services and Hardware Design and Development, where AI-assisted iteration results in performance improvements and lower prototyping costs.
Predictive Analytics in R&D
One of the most powerful contributions of AI is to reduce late-stage development failure.
Using predictive modeling, AI anticipates:
- Biological response scenarios
- Risk factors
- Device failure conditions
- Therapy effectiveness variation
Companies like Insitro leverage AI models to forecast outcomes earlier, lowering the chance of costly failure during clinical stages. For product teams, this transforms how risk, safety, and performance forecasting are managed.
Automated Diagnostics and Clinical Decision Support
AI has also become central to automated diagnostics integrated directly into devices.
GE Healthcare’s Caption AI enables cardiac ultrasound imaging support, guiding clinicians, even those with limited sonography training, in acquiring high-quality images. This represents the convergence of:
- AI algorithms
- Embedded systems
- Smart imaging devices
- Regulatory-grade accuracy
This demonstrates where Embedded Product Development Services and Medical Device Hardware Design intersect with AI-powered clinical intelligence.
Real-time Learning After Product Launch
Modern devices no longer remain static once deployed.
AI enables continuous post-launch learning by:
- Monitoring device behavior
- Identifying performance anomalies
- Improving algorithm accuracy
- Personalizing treatment over time
Eko Health’s AI-driven stethoscopes identify heart murmurs and improve detection accuracy as more patient data is gathered, while still preserving privacy and regulatory constraints.
MedTech is moving toward self-learning devices, capable of improving long after manufacturing, a fundamental shift in lifecycle management.
Where AI Must Go Next: From Add-On Feature to Core Architecture
Today, AI is still too often treated as a patchwork add-on, a feature inside a device, a software enhancement, or a data platform layer.
The next generation of MedTech requires AI to be embedded into the heart of design.
AI-powered Engineering Design Automation
AI can already support:
- Schematic optimization
- PCB routing suggestions
- Thermal behavior simulation
- Failure-mode prediction
- Tolerance optimization
In Hardware Design and Development, AI will:
- Automate low-level design tasks
- Support rapid DFM (Design for Manufacture) decisioning
- Accelerate compliance documentation
- Generate validation test cases
This increases engineering capacity while maintaining design rigor.
Intelligent Electronic Product Design Services
Electronic products in healthcare are no longer isolated devices. They are nodes in connected ecosystems of sensors, mobile apps, clinician dashboards, and cloud analytics.
AI strengthens this ecosystem by enabling:
- Adaptive power management
- Anomaly detection in signals
- Image and waveform interpretation
- User-behavior learning
- Predictive maintenance
Device intelligence shifts from being reactive to proactive.
AI-enhanced Medical Device Hardware Design
Safety-critical devices require accuracy, repeatability, and lifecycle stability.
AI supports Medical Device Hardware Design through:
- Virtual prototyping
- Risk-aware component selection
- Automated traceability and documentation
- Compliance prediction against IEC, ISO, and FDA standards
It does not bypass regulatory diligence; it strengthens it. AI allows MedTech developers to provide stronger evidence, deeper test coverage, and better safety analysis.
Embedded AI: Merging Software Intelligence and Physical Hardware
The future of healthcare devices will be defined by embedded intelligence.
AI is increasingly deployed:
- In edge processors on the device
- In low-power chipsets
- In microcontroller firmware
- In cloud inference layers connected to devices
This is the core domain of Embedded Product Development Services.
AI at the edge enables:
- Faster decisions without cloud latency
- Greater data privacy protection
- Operation in connectivity-limited environments
- Lower bandwidth costs
Think of implantable, wearables, infusion pumps, surgical systems, and remote monitoring devices that analyze data as it is generated rather than uploading it raw.
Post-Launch AI: The Product Lifecycle That Never Truly Ends
Traditional product development assumed that once a device shipped, the design was “finished.” AI breaks this paradigm.
Now, devices continue to:
- Learn from field usage
- Receive OTA algorithm updates
- Improve detection accuracy
- Adapt to patient populations
AI transforms post-market surveillance into active lifecycle intelligence. Manufacturers can detect early safety signals, user interaction patterns, and unexpected performance outcomes much earlier, reducing recalls and improving care quality.
Pinetics: Enabling AI-Driven MedTech Product Innovation
At Pinetics, we help organizations move beyond experimentation and embed AI strategically throughout product development. Our expertise spans:
- Embedded Product Development Services
- Hardware Design and Development
- Electronic Product Design Services
- Medical Device Hardware Design
We work across the entire lifecycle:
- Concept feasibility and product definition
- AI-powered design and simulation
- Embedded system development
- Verification, validation, and regulatory support
- Post-launch analytics and device updates
Our teams bring cross-domain engineering capability across electronics, embedded systems, data science, and clinical technology, allowing AI to become an integrated foundation rather than a disconnected add-on.
Will AI Transform Your Product Development or Someone Else’s?
The real question today is not whether AI will reshape MedTech. That outcome is certain.
The question is whether your organization will:
- Adopt AI end-to-end in product strategy
- Continue experimenting while competitors scale AI-driven innovation
Those who move first will design safer, smarter, more adaptive devices and deliver better patient outcomes at lower cost.
The future of MedTech belongs to organizations that treat AI not as a technology experiment, but as an engineering imperative.
 Sr. Test Engineer
Sr. Test Engineer Sales Marketing Manager
Sales Marketing Manager Marketing & Sales – BBA : Fresher
Marketing & Sales – BBA : Fresher